New Delh: May 26, 2020. Coronavirus (Covid-19) Vaccine Latest Update: While US biotechnology company Moderna Inc and China’s Cansino Biologics Inc may have stolen a march in development of a Covid-19 vaccine, reporting successful results, US firm Novavax has begun injecting a vaccine candidate into people in Australia on Tuesday with hopes of releasing a proven cure this year.
With Covid-19 infecting more than 5.5 million and killing 346,326 people across the world, according to Johns Hopkins University tracker, the US is planning a massive testing effort involving more than 100,000 volunteers and half a dozen of the most promising vaccine candidates to deliver a safe and effective jab by the end of 2020, Reuters reported.
Meanwhile, what buttresses the fact of India’s growing role in development for a cure for Covid-19, French Ambassador Emmanuel Lenain has said the country had an important hand to play in the mass production of vaccines.
For Covid-19, there are over 100 vaccines being developed across the world now, some from scratch, some from existing molecules developed for other diseases. Most of the experimental vaccines in progress aim to train the immune system to recognize the “spike” protein that studs the coronavirus’ outer surface, priming the body to react if it was exposed to the real virus. There are at least 10 vaccines in human trials, according to the World Health Organization (WHO).
Coronavirus (Covid-19) vaccine latest updates:
? US biotechnology company Novavax has become the latest to announce that it has started human trials of its NVX-CoV2373 vaccine for Covid-19 in Australia, AP reported. Novavax will inject 131 volunteers in the first phase of the trial, the company’s research chief Dr Gregory Glenn said.
“The trial began with six volunteers being injected with the potential vaccine in Melbourne on Tuesday,” said Paul Griffin, infectious disease expert with Australian collaborator Nucleus Network.
The results of the first phase of clinical trials in Melbourne and Brisbane are expected to be known in July following which thousands of candidates in several countries would then become involved in a second phase.
Animal testing suggested the recombinant vaccine is effective in low doses. Novavax is planning to manufacture at least 100 million doses this year and 1.5 billion in 2021.
Novavax uses genetic engineering to grow harmless copies of the coronavirus spike protein in giant vats of insect cells in a laboratory. Scientists extracted and purified the protein, and packaged it into virus-sized nanoparticles.
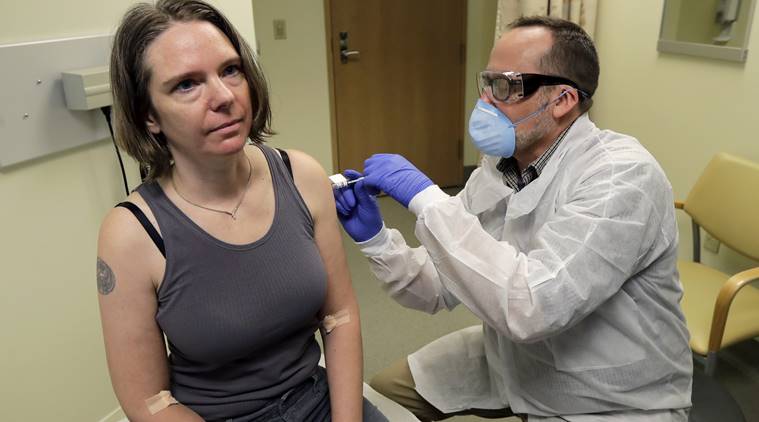
A pharmacist gives a woman the first shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19. (AP)
? Coming to India, the Indian Council of Medical Research (ICMR) told news agency IANS that human trials of its Covid-19 vaccine might begin in six months. The ICMR and Bharat Biotech International Limited (BBIL) are jointly developing the vaccine.
“The virus strain isolated at the National Institute of Virology (NIV) laboratory in Pune will be used to develop the vaccine, and this strain has been successfully transferred to BBIL. It is expected that the human trials of the vaccine will begin in at least six months,” Dr Rajni Kant, Director Regional Medical Research Centre and Head at ICMR said.
ICMR and BBIL are working towards developing a killed virus vaccine that usually provides good immunogenicity. By entering the body, it will create antibody against the infection. Presently, while Zydus Cadila is working on two vaccines, Serum Institute, Biological E, Bharat Biotech, Indian Immunologicals, and Mynvax are developing one vaccine each.
? Meanwhile, as part of its “Operation Warp Speed”, the US plans a massive testing effort involving more than 100,000 volunteers and 14 vaccine candidates, Reuters reported. The project will compress what is typically 10 years of vaccine development and testing into a matter of months.
To achieve this, leading vaccine makers have agreed to share data and lend the use of their clinical trial networks to competitors as a part of a public-private partnership called Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV).
Between 100,000 and 150,000 people will be enrolled in the studies, said Dr Larry Corey, a vaccine expert at Fred Hutchinson Cancer Center in Seattle, who is helping design the trials. Vaccines by Moderna, Oxford University and AstraZeneca Plc, Johnson & Johnson, Sanofi and Merck and Co are likely to be included.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” said Dr Anthony Fauci, director of the National Institutes of Allergy and Infectious Diseases at NIH.
Thailand, which has entered the race with its Thai mRNA vaccine, recently said its aim is to make it cost-effective and accessible to Southeast Asia and play a part in preventing a supply shortage globally, Reuters reported.
“We don’t aim for making money. It’s not a money issue but an accessibility one,” said Kiat Ruxrungtham, director of Bangkok’s Chulalongkorn University’s coronavirus vaccine development. Trials of the experimental vaccine using monkeys started last week after researchers successfully conducted trials on mice.
The University has partnered with scientists and biotech companies in North America and wants to mass produce the vaccine in Thailand, at a price more affordable there and in nearby markets like Indonesia, Malaysia, Laos, Vietnam and Myanmar.
“If our neighbours still have high infection numbers then we won’t survive as well in the long term,” Kiat said.
? Japanese biopharma venture AnGes Inc said it would begin clinical trials for its DNA vaccine in July, earlier than previously planned, according to a report in The Japan Times. The news saw shares jumping as much as 19 percent in Tokyo trading, taking its gaining streak to a fourth day.
In a statement, AnGes said animal trials of the vaccine it had been conducting with Osaka University since March had confirmed an increase in antibodies. “We will examine the results of the toxicity data and swiftly move forward to clinical trials,” AnGes said.
DNA vaccines are a type of vaccine that use genetic material from pathogens to produce antibodies by inducing an immune system response. While such a vaccine has yet to be approved for human use, the technology has been touted by experts as safe and one that can ease large-scale manufacturing.
If the effectiveness is proven, the vaccine may receive approval by the end of the year, Nikkei Asian Review said in a report.